Abstract
Introduction
Acute myeloid leukemia (AML) is a clonal disease with a reduced life expectancy due to a high relapse rate. One explanation is that leukemic stem cells (LSC) evade the action of conventional chemotherapy due to their quiescent state. Several mechanisms have been proposed that regulate their quiescence, however, by analogy with normal hematopoietic stem cells, a key role may be carried out by the signaling pathways Notch and Hedgehog (Hh). The objectives of this study are to analyze the role of Notch and Hh pathways in the quiescence of LSC and to verify if the pharmacological inhibition of the Notch and Hh pathways decreases the percentage of quiescent LSC. In this way, LSCs would be sensitized to chemotherapy treatments and the high relapse rate of AML could be reduced.
Methods
Expression of GLI1, a transcription factor of the Hh signaling pathway and NOTCH Internal Cleaved Domain (NICD) were analyzed in the hematopoietic stem and progenitor cells of four patients diagnosed with AML. The selection of quiescent fraction was performed by flow cytometry using anti-CD34-FITC, anti-CD117-PerCP, anti-CD45PE-Cy7, anti-CD38-APC-Cy7 and anti-KI67-BV510 antibodies. KI67 negative cells were considered quiescent. The activation of NOTCH and Hh pathways was studied using rabbit anti-GLI, anti-NICD primary antibodies and anti-rabbit BV421 secondary antibodies. Results were expressed in median (range) or mean ± standard deviation.
Dose-response curves of inhibitors of the Notch pathway (BMS-906024, inhibitor of γ-secretase), Hh pathway (BMS-833923, SMO inhibitor and GANT61, GLI inhibitor), and cytarabine (AraC) were made to study drug potency in the OCI-AML3 cell line. We also analyzed its synergistic behavior in combination with Arac by calculating the combination index (CI) of each of them. These experiments were conducted in triplicate and values were expressed as the mean ± standard deviation.
Finally, the effect of BMS-833923 and BMS-906024 on the quiescence of the CD34+CD38- cells of two patients diagnosed with AML was studied by flow cytometry.
The paired samples t-test was used in the statistical analysis of GLI and NICD expression between G0 and proliferating cells and in the statistical analysis of the decrease of quiescent cells due to Notch and Hh inhibitors.
Results
First of all, hematopoietic and progenitor cells were quantified in four AML patients: the median of the percentage of CD34+CD38- cells with respect to total cells in the bone marrow of the AML patient studied was 1.1% (range: 0.12%-9.05%), within which 71.50% (range: 64.30%-88.43%) are quiescent. Interestingly, we found a trend for a higher expression of NOTCH signaling pathway in the proliferating CD34+CD38- cells (relative median fluorescent intensity (MFI) = 1.91 (range: 1.51-3.34)) compared to the quiescent fraction (relative MFI=1.55 (range: 1.18-1.94); p=0.105). But no differences were found in expression of GLI1.
Before studying the effect on cellular quiescence of Notch and Hh inhibitors in monotherapy and in combination with AraC, we evaluated their effect on cell viability. The most potent drug studied was AraC (IC50 = 4.055 μM), followed by inhibitors of the Hh pathway (IC50 BMS-833923 = 5.041 μM; IC50 GANT61 = 7.042 μM) on the OCI-AML3 cell line. In contrast, the γ-secretase inhibitor (BMS-906024) showed no effect. Moreover, it was found that the combination 0.8 μM AraC plus 8 μM BMS-833923 was the most synergistic (CI = 0.53, 15% viability with respect to DMSO control).
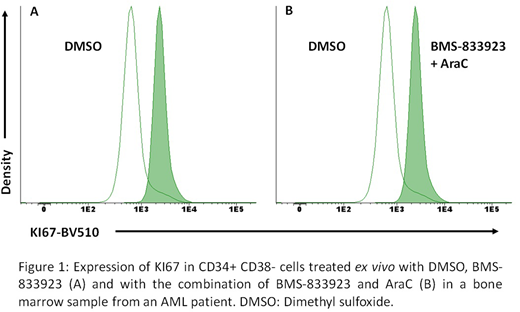
Subsequently, the effect of the SMO inhibitor on the quiescent CD34+CD38- cells of two patients diagnosed with CD34+ AML was analyzed: BMS-833923 decreased the percentage of quiescent CD34+CD38- cells by 88.5±16.3% in monotherapy (p=0.083) and in presence of AraC by 85.8 ± 21.2% (p=0.113) (figure 1).
Conclusion
The use of SMO inhibitors for the treatment of AML is promising because it increases the sensitivity of leukemic cells to chemotherapy and facilitates their action by reducing the percentage of quiescent LSC. This could mean a decrease in the probability of relapse in patients with AML treated with Hh inhibitors. These results were derived from an ongoing project and more patients are being studied in order to confirm the explained results.
This work is partially funded by the Madrid Association of Hematology and Hemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.